Mentor Search
- Institution:University of Nevada, Reno
- Departments: Pharmacology, Ob/gyn
- Research Fields: Pregnancy, Preterm Labor, Myometrium, Smooth Muscle, Breast Cancer, Drug Development
- Disciplines: Biochemistry, Biological and Biomedical Sciences, Cell/Cellular and Molecular Biology, Human/Medical Genetics, Molecular Pharmacology, Pharmacology, Pharmacology and Toxicology
- Location:Washoe County
- Funding:NIH - National Institutes of Health
Mentoring
In the forty years I have been a research scientist, I have trained fifteen students as their PhD advisor. In this time I have also mentored a similar number of postdoctoral fellows. Every year we have undergraduate students in the lab and many of these young people are now successful as physicians and scientists in their own right. I have mentored over 80 undergraduate students and many have been winners of local research poster and short talk competitions. Successful students are able to attend national and international research meetings.
Biography
The Buxton lab is exploring contraction-relaxation coupling in the uterine myometrium in order to better understand and develop treatments for the problem of preterm labor. Preterm delivery of an underdeveloped fetus is a global problem. Babies delivered prior to full development at term have multiple medical problems that plague these individuals throughout their lifetime. Prematurity explains 75% of all fetal morbidity and mortality. Thus, beyond the tragic and costly fact of their prematurity, is the major impact on individuals and societies long-term. There are no effective (or FDA-approved) medications that prevent contractions of the uterus in patients who enter labor preterm (PTL). What is used is ineffective at allowing the fetus to remain in the womb until term. Drugs employed to prevent PTL (tocolytics) are only evaluated for an ability to prevent labor for 48 hours, a time during which treatments can ready the fetus to breath air. PTL leads to preterm delivery (PTD) in over 50% of cases. Spontaneous PTL (sPTL, no explanation such as infection) accounts for the majority of PTL.
The approach to sPTL we are pursuing is based on the non-canonical pathway by which NO relaxes myometrium. Our approach hypothesizes specific S-nitrosation differences in the protein fingerprint of sPTL compared with laboring myometrium. What is needed to investigate sPTL is to know the specific proteins that are post-translationally S-nitrosated and their abundance and/or unique presence and the impact of their S-nitrosation in pregnancy, labor and sPTL.
We have discovered particular unique proteins that are deferentially S-nitrosated and are pursuing their role in mediating relaxation on pregnancy and labor. One such protein is a channel called TREK-1. This channel is stretch-activated. We discovered genetic variants of the channel associated with PTL in women. Electrophysiological measurement of these gene variant channels suggests that their expression in women may constitute a mechanism to explain PTL in these patients. Drug discovery is in process to generate therapeutics to treat this form of PTL.
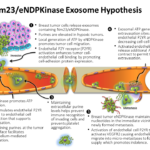
In a second thrust, the Buxton lab is looking for therapeutic targets in breast cancer. Tumor cells migrate to distant sites in the body before they are capable of forming aggressive metastases and thus remain dormant. We do not know the cellular behavior of disease we label latent but attracting a blood supply may be an early property that precedes and is required for those lesions that become malignant in women. Breast cancer specific mortality is almost exclusively a function of metastasis. Growth of tumor cells as metastases dictates that tumor cells must first develop a capillary blood supply or risk necrosis. Metastatic tumor cells have already attracted a blood supply, a hallmark of cancer. What activates dormant cells at metastatic sites to move from a quiescent to aggressive phenotype is not known. It is critical to determine the effect of a kinase we discovered to be released from cancer cells because every indication is that it produces a blood supply for cells that can later become malignant, an event that cannot take place unless a blood supply is available. Our current experiments are focused on the actions of the kinase that permit intravasation and extravasation of tumor cells that permit their passage to distant sites in the body where they can lodge and remain undetected for years. We have developed an inhibitor of the kinase and hope to demonstrate its potential a breast cancer prophylactic.